Copper has some outstanding properties which make it very desirable in many industries. Two of which are ductility and strength. However, in order to get the most out of those two properties, the material needs to be processed rightfully.
Ductility, as well as strength, are two quite important and desirable properties in copper alloys. Copper and its alloys are characterized by their high ductility and comparatively high strength. This combination, as well as other typical copper properties such as conductivity, corrosion resistance, or machinability, makes copper suitable for many different applications. However, this blog post deals specifically with the relationship between ductility and strength.
What is ductility?
Ductility is the property of materials to deform plastically under load before failure occurs (for example through fracture). The material hereby always retains a constant volume. Ductility varies depending on the material: while glass breaks without noticeable deformation, steel can be deformed by more than 25 % before it cracks. If material is hardly deformable, such as glass, it is called brittle.
There are two measures used for ductility: elongation and reduction of area:
- Elongation is reported as a percent increase in the original sample gauge length and is measured after the sample fracture.
- Reduction of area is reported as a percent decrease in the original cross-sectional area and is measured after the sample fracture.
While elongation is gauge length-dependent, reduction of area is not.
What is strength?
The strength of a material describes its ability to withstand mechanical stress before failure – such as unintentional bending or breaking – occurs. The strength indicates the maximum stress that a material can withstand during its deformation. Typically, alloys achieve higher strength than pure metals. Materials with high strength are particularly suitable for lightweight construction but are generally difficult to machine.
As you may have noticed, strength stands in direct contrast to ductility, which results in a strength-ductility trade-off dilemma. In materials science, this dilemma describes that in order to increase the strength of a material, the ductility inevitably decreases and vice versa. Let’s take a look at some of the most popular methods in order to strengthen the material.
Strengthening due to restriction of dislocation motion
- Solid solution hardening: In solid solution strengthening, the mechanical properties of solids are changed by the incorporation of interstitial or substitutional atoms. In the process, other metallic elements, such as beryllium, tin, aluminum, or nickel, are added to copper. These ensure that the dislocation movement is impeded and thus ultimately the strength increases. Different elements have a varying influence on the increase in strength. See on the graph below the effects of several alloying elements on the yield strength of copper.
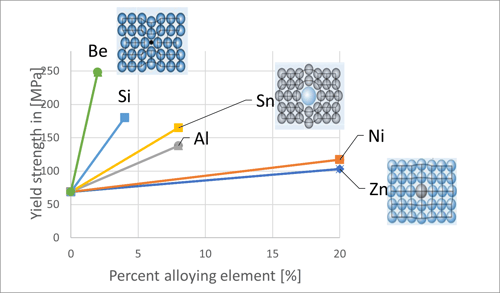
- Cold forming: In cold forming, metals are formed below the recrystallization temperature. In the process, the grains tend to elongate in the direction of deformation. Consequently, the average distance between the dislocations decreases, which blocks the movements. Larger deformations increase the strength of the alloy, but at the same time reduce its ductility. The result is greater strength with lower ductility. The picture below illustrates the effects of cold forming using AMPCOLOY® 83 as an example.
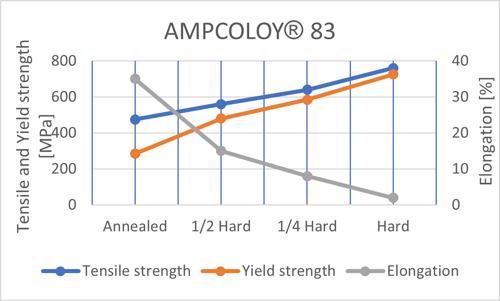
- Grain size reduction: In grain size reduction, a finer, smaller grain is produced in the structure by suitable heat treatment or seeding of the melt. Although the effect on strength is rather small, copper alloys will be stronger with smaller grain sizes. This is because smaller grains have a greater ratio of grain boundary and the more grain boundaries, the higher the strength. The graph below illustrates the relationship of grain size on tensile strength and elongation for a strip in brass.
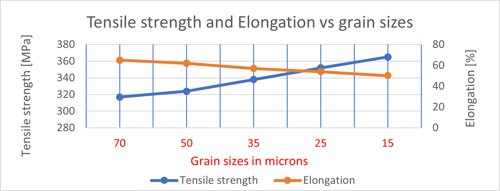
Increase both: strength and ductility
During precipitation hardening, the smallest possible, evenly distributed particles are formed. For this purpose, the alloy is heated until all elements necessary for precipitation are dissolved. The alloy is then quenched to prevent diffusion and the alloy atoms remain supersaturated in the single-phase solid solution. The material is then tempered again to achieve controlled diffusion. Precipitates are formed which serve as obstacles against the dislocation movements. Thus, the processed material has high strength while maintaining ductility.
Therefore, precipitation hardening has proven to be the most adequate way to achieve the optimum combination of strength and ductility in copper-based alloys. For example, AMPCOLOY® 940 and AMPCOLOY® 972.
You want to know more about the metallurgy of copper and copper-based alloys? Download now a free extract of our book «Metallurgy of Copper and Copper Alloys»





